Avive+ Soft Tissue Matrix™
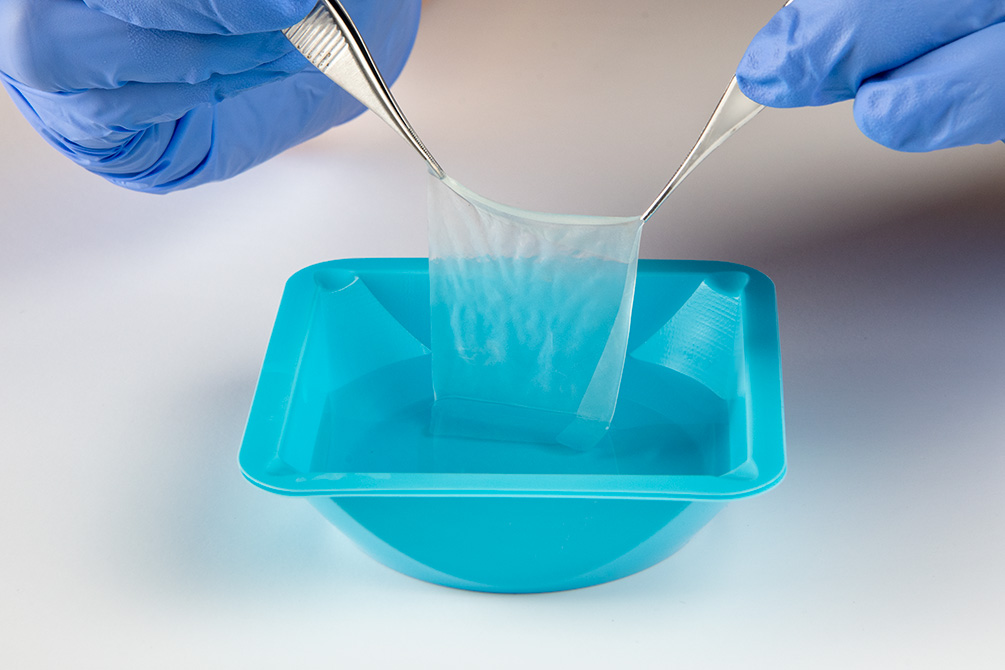
Avive+ Soft Tissue Matrix™ features multi-layered amniotic tissue that serves as a barrier for protection during the critical stage of healing, making it the ideal solution for acutely traumatized nerves and soft tissue.1
Protection when it's needed most
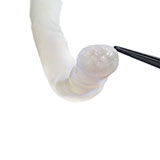
- Keeps adjacent tissues separated and acts as a barrier to soft tissue attachments, which may reduce the potential of nerve tethering and impaired nerve function.1
- Present for at least 16 weeks during the critical phase of tissue repair, as confirmed in an animal study model.1
- Multi-layered design allows for ideal handling, easy repositioning, and the ability to suture or secure into place.1
- Unique, layered design features epithelial cells on both sides.1
- to order, obtain more information or report adverse events
contact axogen customer care: - Phone
888.axogen1 (888.296.4361) - Email
customercare@axogenInc.com - see sizing info below
Where to incorporate Avive+ into surgical practice
Where I found an Avive+ Soft Tissue Matrix to fit in my practice is in areas where I want early motion and I’m concerned about adhesions.
— MEREDITH N. OSTERMAN, MD
Considerations for amnion in nerve surgery
Having that barrier and that protection of this amniotic product, I think is a game changer in my practice for decreasing the sensitivity. And when I use an Amnion product, I actually feel more confident that I’m going to get a patient’s response improved, especially if they’re coming in hypersensitive, which many nerve injuries lead to that.
— AMY MOORE, MD, FACS
REGULATORY CLASSIFICATION: Avive+ Soft Tissue Matrix is processed and distributed in accordance with U.S. FDA requirements for Human Cellular and Tissue-based Products (HCT/P) under 21 CFR Part 1271 regulations, and U.S. State regulations.
Axogen Corporation is accredited by the American Association of Tissue Banks (AATB).
INTENDED USE: Avive+ Soft Tissue Matrix is intended for use as a soft tissue barrier. The allograft may be used in numerous clinical applications, including covering the peripheral nerve to separate and protect the nerve from the surrounding environment.
Rx Only The allograft is to be dispensed only by or on the order of a licensed health professional.
Each allograft is intended for single-patient use.
CONTRAINDICATIONS: Avive+ Soft Tissue Matrix is contraindicated for use in any patient in whom soft tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing or evidence of a current infection.
Package Insert
resources
Reimbursement
| Code | Dimensions |
| CA2020 | 2 cm x 2 cm |
| CA2040 | 2 cm x 4 cm |
| CA3060 | 3 cm x 6 cm |