for small-gap repair,
new evidence challenges primary repair as the universal gold standard
The answer is yes: Connector-Assisted Repair® (CAR) leads to a higher proportion of patients achieving meaningful recovery (MR).1
The historical standard of peripheral nerve repair has been direct repair, also called primary repair, of two nerve ends in proximity; however, suboptimal outcomes often occur.1,2
A 2024 meta-analysis showed that using a CAR technique resulted in significantly better sensory MR rates—even at higher-threshold MR standards.1
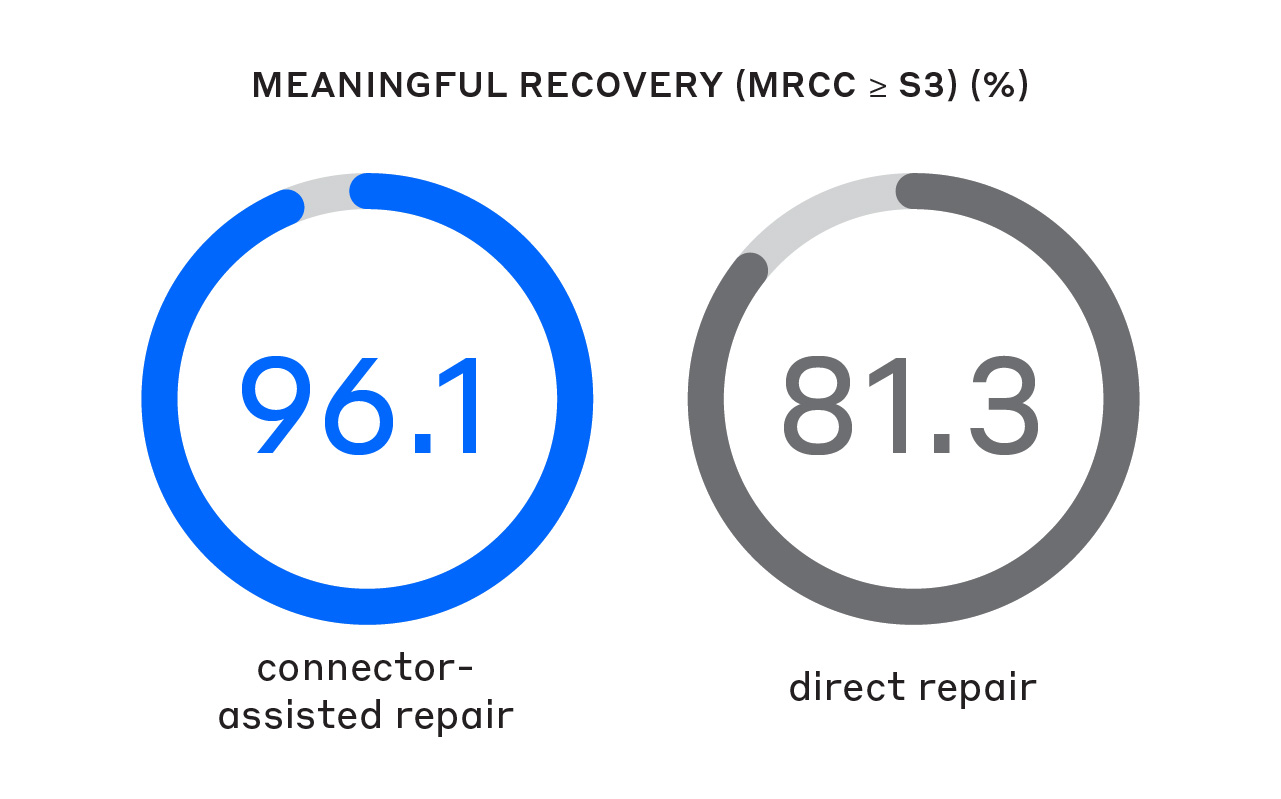
A significantly greater proportion of patients achieved MR (Medical Research Council Classification [MRCC] ≥ S3) compared to direct repair.1
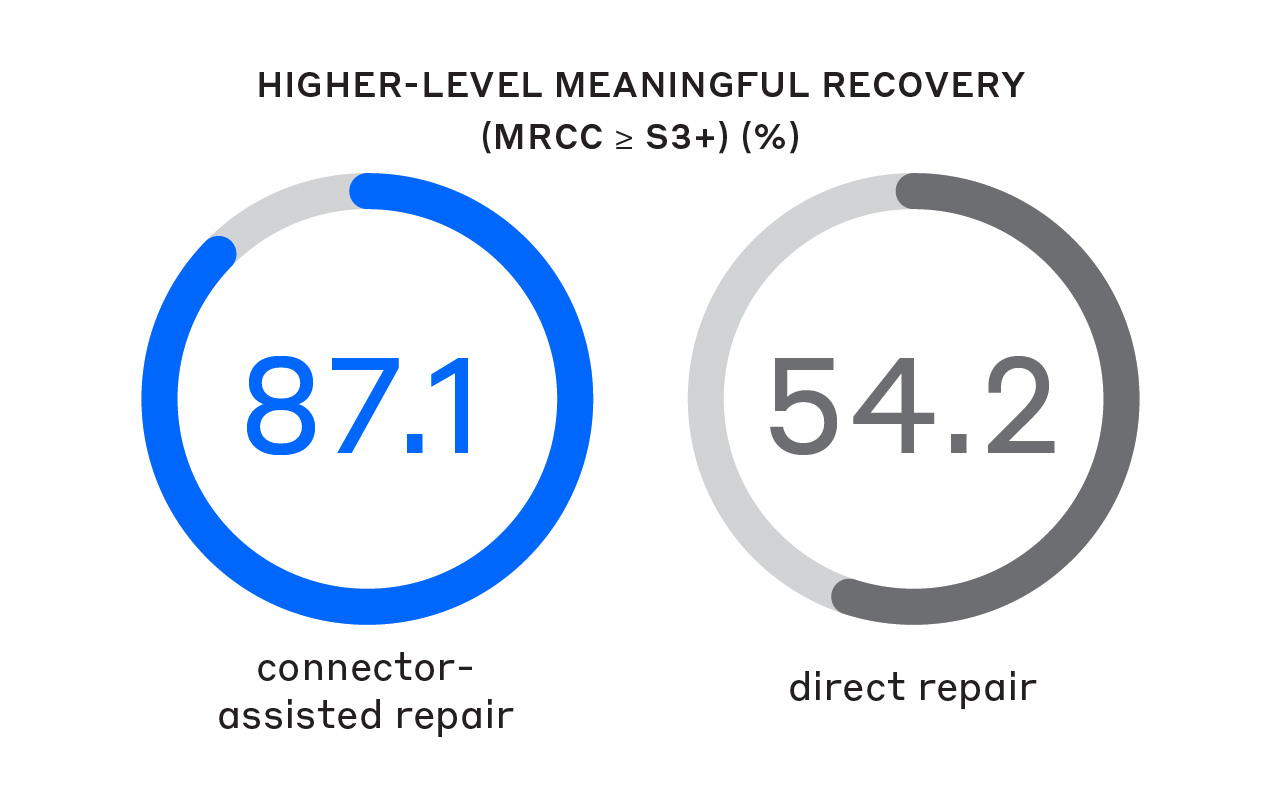
A significantly greater proportion of patients achieved higher-level MR (MRCC ≥ S3+) compared to direct repair.1
Patient follow-up after surgical intervention is challenging, especially for nerve-related injuries, due to inconsistent patient compliance with post-surgical rehabilitation plans.
Because of this, reinforcing a suture-only direct repair with a coaptation aid or protector can help to safeguard potentially non-compliant patients from poor functional recovery or even failure.
(Mean = 15%)
Results based on a self-reported survey of 150 hand fellowship-trained surgeons4
“What percent of your patients that have primary/direct repair have the repair separate before it is healed and require a revision surgery?”
Base: N = 272 (2018 n=122, 2022 n = 150)
Repair under tension can lead to misaligned fascicles, repair rupture,6 axonal escape and resultant neuroma formation.3 As little as 7–8% elongation or stretching of the nerve due to tension results in a 50% decrease in blood flow and an almost 30% reduction in axonal outgrowth.7,8
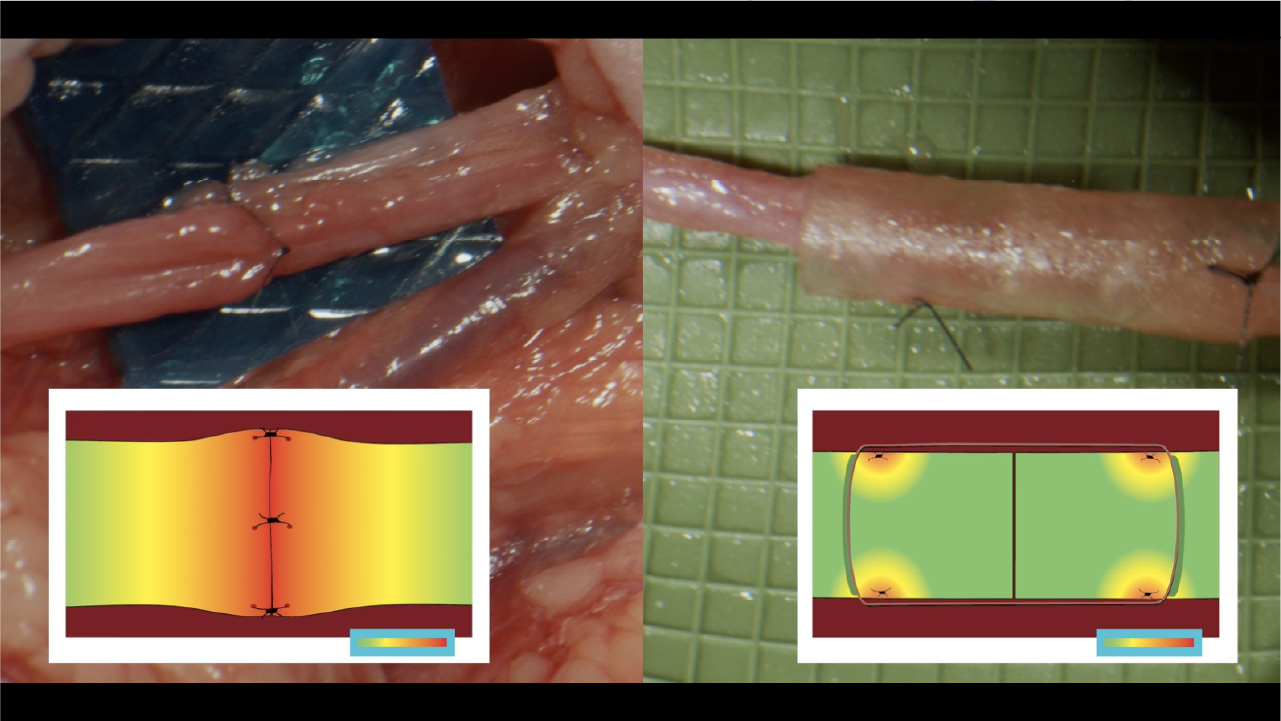
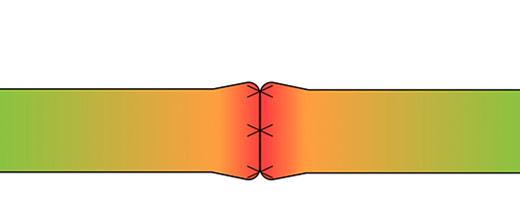
Normally, tension is distributed along the length of the nerve. Once that nerve is transected, the proper tension distribution cannot be restored as tension becomes concentrated at the repair site.5 By allowing suture placement away from the coaptation, CAR minimizes tension and reduces the risk of ischemia.1
In addition, CAR makes it easier to achieve better alignment of the nerve ends,3 which reduces the risk of forced fascicular mismatch and neuroma formation.1
direct repair
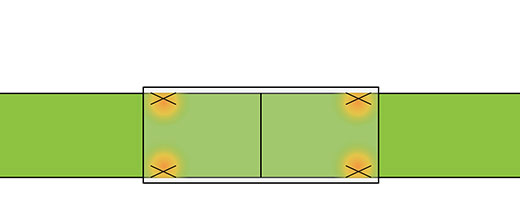
Tension map highlighting the localized tension at the coaptation site in direct repairs5
connector-assisted repair
Tension map highlighting tension concentrated away from the coaptation site with a CAR5
Nerve repair techniques that off-load tension at the coaptation site can allow patients to begin mobility exercises sooner. Early mobilization is particularly important when there is a concomitant tendon injury, which data shows may occur in up to 40% of digital nerve injuries.9
In patients with acute limb injuries, early mobilization decreases pain and swelling and improves functional outcomes compared with cast immobilization.10
Primary repair concentrates tension at the critical zone of regeneration. CAR reduces tension in this critical zone allowing for early range of motion while decreasing the chance for repair failure.5
PlayWhen a nerve is cut, the mechanism may have a significant impact on the extent of the zone of injury.
“Clean” cuts, like a glass or knife injury, are sometimes considered to be ideal candidates for suture-only direct repair. However, even in these cases, the zone of injury extends beyond what is easily observable upon examination.
Research suggests that a nerve should be stabilized and transected with a thin sharp blade.11 It has been demonstrated that both sharp scissors and a no. 11 scalpel blade show significantly less surface area roughness compared to a razor blade, despite what may otherwise appear as a “clean” cut.12
These images show scanning electron microscope (SEM) images of nerves cut by a sharp piece of glass and fresh kitchen knife in comparison to nerve trimmed with a fresh surgical scalpel, demonstrating the impact that even the sharpest injury mechanisms can have on surrounding nerve tissue.13
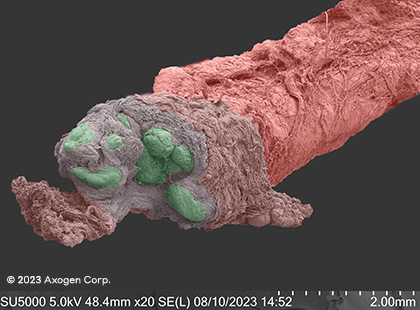
glass*
Dry SEM
Ulnar nerve at wrist level
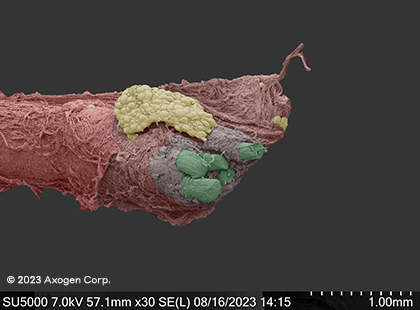
kitchen knife*
Dry SEM
Common digital nerve
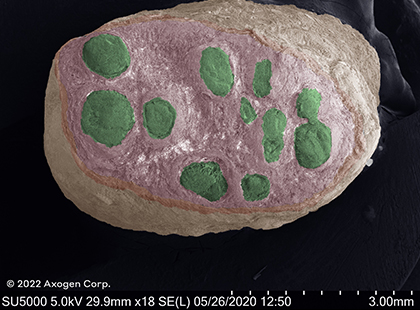
surgical cut*
Cryo SEM
Common digital nerve
*red: epineurium, green: fascicles, yellow: connective tissue
CAR makes it easier to achieve a better repair and more consistent outcomes than direct repair.1,3
See sizing and IFU on solution pages.


A semi-translucent coaptation aid designed for CAR of a transected nerve with a gap up to 5 mm.15

![]()
A remodeling extracellular matrix for long-term protection with a short-term gel coating enhances nerve gliding and minimizes soft tissue attachments.16
connect with a nerve rep
There’s only a short form between you and our nerve product team who can help you get more information about our nerve repair solutions.
explore educational events
Axogen offers a robust variety of events and opportunities for you to interact with experts and stay up to date on the most recent happenings in peripheral nerve surgery. Learn more about and practice techniques related to Connector-Assisted Repair.
references
- Leis A, et al. Comparative effectiveness systematic review and meta-analysis of peripheral nerve repair using direct repair and connector-assisted repair. Plast Reconstr Surg Glob Open. 2024;12(7):e5927. doi:10.1097/GOX.0000000000005927
- Jain SA, et al. Clinical outcomes of symptomatic neuroma resection and reconstruction with processed nerve allograft. Plast Reconstr Surg Glob Open. 2021;9(10):e3832. Published 2021 Oct 4. doi:10.1097/GOX.0000000000003832
- Isaacs J, et al. Technical assessment of connector-assisted nerve repair. J Hand Surg Am. 2016;41(7):760-766. doi:10.1016/j.jhsa.2016.04.015
- Axogen, Inc. Data on File. Document Reference Number 001. 2024.
- Jewell E. Nerve de-tensioning effects of conduits: a quantitative cadaveric analysis comparing conduit-assisted and direct digital nerve repairs. Paper presented at: AAHS 2023 Annual Meeting; January 17-21, 2023; Miami, FL.
- Brogan DM, et al. Influences of repair site tension and conduit splinting on peripheral nerve reconstruction. Hand (N Y). 2022;17(6):1048-1054. doi:10.1177/1558944720974117
- Clark WL, et al. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg Am. 1992;17(4):677-687. doi:10.1016/0363-5023(92)90316-h
- Yi C, et al. Impaired nerve regeneration and Schwann cell activation after repair with tension. Neuroreport. 2010;21(14):958-962. doi:10.1097/WNR.0b013e32833e787f
- Evertsson L, et al. Incidence, demographics and rehabilitation after digital nerve injury: A population-based study of 1004 adult patients in Sweden. PLoS One. 2023;18(4):e0283907. Published 2023 Apr 7. doi:10.1371/journal.pone.0283907
- Nash CE, et al. Resting injured limbs delays recovery: a systematic review. J Fam Pract. 2004;53(9):706-712.
- Smetana B, et al. Improving nerve end preparation for neurorrhaphy through use of a circumferentially constraining surgical device. Poster presented at: American Association for Hand Surgery Annual Meeting; January 10-13, 2018; Phoenix, AZ.
- Rose RA, et al. The cutting edge: Surface texture analysis following resection of nerve stumps using various instruments. Plast Reconstr Surg Glob Open. 2021;9(5):e3566. doi:10.1097/GOX.0000000000003566
- Axogen, Inc. Data on File. Document Reference Number 002. 2024.
- Axogen, Inc. Data on File. Document Reference Number 003. 2024.
- Axoguard Nerve Connector US Prescribing Information, Alachua FL: Axogen Corporation; 2024.
- Axoguard HA+ Nerve Protector US Prescribing Information, Alachua FL: Axogen Corporation; 2024.