Avance Nerve Graft®
Avance Nerve Graft is the only off-the-shelf biologically active processed human nerve allograft intended for the surgical repair of peripheral nerve discontinuities.
Avance innovations
- Organized, linear and continuous structural support for cellular migration and regenerating axons.
- Clinically proven, off-the-shelf solution with 82% meaningful recovery† in sensory, mixed and motor nerve gaps up to 70 mm.1
- Proprietary cleansing, decellularizing and sterilizing methods specifically designed to maintain both the natural nerve structure and biological activity of native nerve.
- Intra-operative versatility to meet a range of anatomical needs.
- to order, obtain more information or report adverse events
contact axogen customer care: - Phone
888.axogen1 (888.296.4361) - Email
customercare@axogenInc.com - see sizing info below
preferred
choice
Avance Nerve Graft is the preferred choice in digital nerve repair over conduit, autograft or vein graft.*2
*Results of a survey of 461 hand surgeons with their method for repairing a 2 cm digital nerve gap
82%
meaningful recovery rate†,
defined as Medical Research Council Scale of S3/M3 or greater, throughout the body after reconstruction.1
14+
years of
unrivaled expertise in nerve tissue recovery.
The Avance Method is Axogen’s proprietary methodology for recovering, cleansing, sterilizing and ensuring quality of the Avance® Nerve Graft. This establishes Avance Nerve Graft as the most effective, natural alternative to a patient’s own tissue.3,4
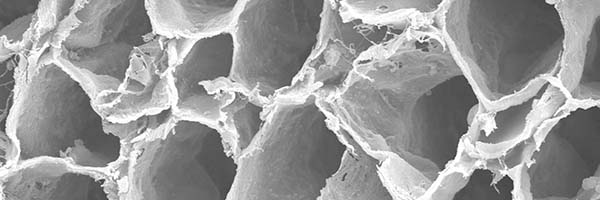
preserved architecture
Preserves the delicate 3D macro- and micro-architecture of native nerve, which provides structural support for the regenerating axons.
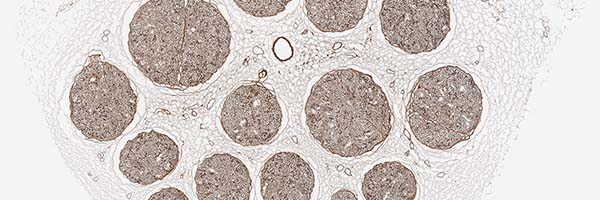
bioactive laminin
Enzyme treated to inactivate the inhibitory aspects of chondroitin sulfate proteoglycans (CSPGs) exposing laminin, which is known to promote nerve regeneration.5
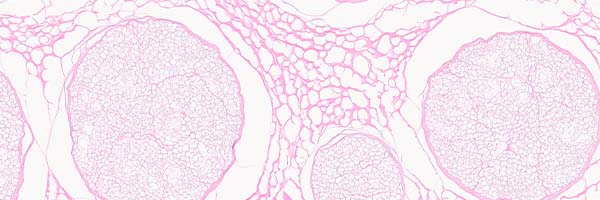
quality assays
Proprietary testing to ensure identity, purity and safety.
REGULATORY CLASSIFICATION: Avance Nerve Graft is a human tissue for transplantation. Avance Nerve Graft is processed and distributed in accordance with US FDA requirements for Human Cellular and Tissue-based Products (HCT/P)under 21 CFR Part 1271 regulations, US State regulations, and applicable international regulations.
Axogen Corporation is accredited by the American Association of Tissue Banks (AATB).
This graft is to be dispensed only by or on the order of a licensed physician.
INDICATIONS FOR USE: Avance Nerve Graft is a processed nerve allograft (human) intended for the surgical repair of peripheral nerve discontinuities to support regeneration across the defect.
CONTRAINDICATIONS: Avance Nerve Graft is contraindicated for use in any patient in whom soft tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing or evidence of a current infection.
reimbursment
| Code | Dimensions |
| 111215 | 1–2 mm x 15 mm |
| 211215 | 2–3 mm x 15 mm |
| 311215 | 3–4 mm x 15 mm |
| 411215 | 4–5 mm x 15 mm |
| 111230 | 1–2 mm x 30 mm |
| 211230 | 2–3 mm x 30 mm |
| 311230 | 3–4 mm x 30 mm |
| 411230 | 4–5 mm x 30 mm |
| Code | Dimensions |
| 111250 | 1–2 mm x 50 mm |
| 211250 | 2–3 mm x 50 mm |
| 311250 | 3–4 mm x 50 mm |
| 411250 | 4–5 mm x 50 mm |
| 111270 | 1–2 mm x 70 mm |
| 211270 | 2–3 mm x 70 mm |
| 311270 | 3–4 mm x 70 mm |
| 411270 | 4–5 mm x 70 mm |