Axoguard Nerve Connector®
Axoguard Nerve Connector is a semi-translucent coaptation aid designed for Connector-Assisted Repair® (CAR) of a transected nerve with a gap up to 5 mm.
connector solutions
- Connector-Assisted Repair alleviates tension and suture-related inflammation at the critical zone of regeneration.2-4
- Connector-Assisted Repair provides an alternative to direct repair that reduces the potential for axonal escape and aiding alignment of nerve ends.
- Axoguard® porcine small intestine submucosa (SIS) vascularizes and remodels into the patient’s own tissue, creating a physical barrier to surrounding soft tissue.5
- Intra-operative versatility aids in reinforcing the coaptation site of direct, graft or cable graft repairs.
- to order, obtain more information or report adverse events
contact axogen customer care: - Phone
888.axogen1 (888.296.4361) - Email
customercare@axogenInc.com - see sizing info below
#1
U.S. market leader*
in off-the-shelf nerve reconstruction solutions.
*IQVIA U.S. market data
50%
increased likelihood of pain
at the coaptation site when primary suture is used versus CAR with various conduits.2
SIS
porcine SIS material
offers excellent flexibility and is semi-translucent compared to opaque collagen conduits.
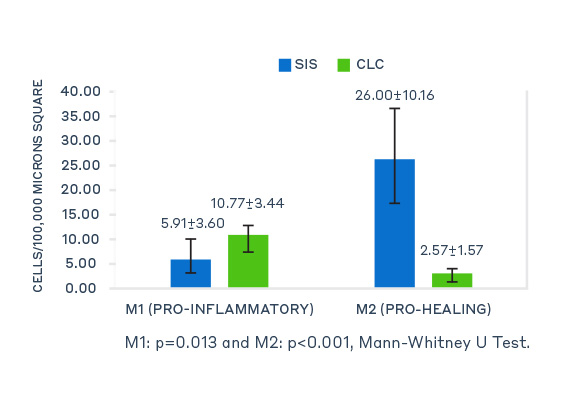
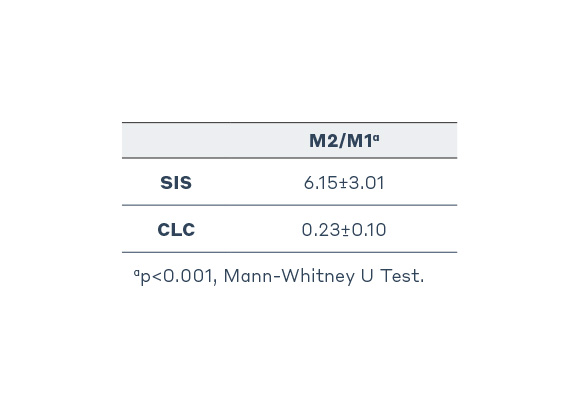
In a pre-clinical study conducted to evaluate host tissue response, porcine small intestine submucosa (SIS, Axoguard Nerve Connector) performed significantly better than cross-linked bovine collagen type I (CLC, Integra NeuraGen®) in all outcomes and categories.
Specifically, the study provided evidence that SIS had increased levels of M2 (pro-healing) macrophages which are indicative of a remodeling response, while CLC had increased levels of M1 (pro-inflammatory) macrophages indicative of a chronic inflammatory response.6,7
M1 and M2 Macrophage Distribution
host inflammatory response
INDICATIONS FOR USE: Axoguard Nerve Connector is indicated for the repair of peripheral nerve discontinuities where gap closure can be achieved by flexion of the extremity. The device is supplied sterile and is intended for one-time use.
CONTRAINDICATIONS: This device is derived from porcine source and should not be used for patients with known sensitivity to porcine material.
| Code | Dimensions |
| AGX110 | 1.5 mm x 10 mm |
| AGX210 | 2 mm x 10 mm |
| AGX310 | 3 mm x 10 mm |
| AGX410 | 4 mm x 10 mm |
| AGX510 | 5 mm x 10 mm |
| AGX610 | 6 mm x 10 mm |
| AGX710 | 7 mm x 10 mm |
| Code | Dimensions |
| AGX115 | 1.5 mm x 15 mm |
| AGX215 | 2 mm x 15 mm |
| AGX315 | 3 mm x 15 mm |
| AGX415 | 4 mm x 15 mm |
| AGX515 | 5 mm x 15 mm |
| AGX615 | 6 mm x 15 mm |
| AGX715 | 7 mm x 15 mm |